Clinical Data
The use of a polycaprolactone based biostimulatory urethral implant for the treatment of female stress urinary incontinence (SUI); a pivotal trial.
The use of a polycaprolactone based biostimulatory urethral implant for the treatment of female stress urinary incontinence (SUI); a pivotal trial.
February 2022
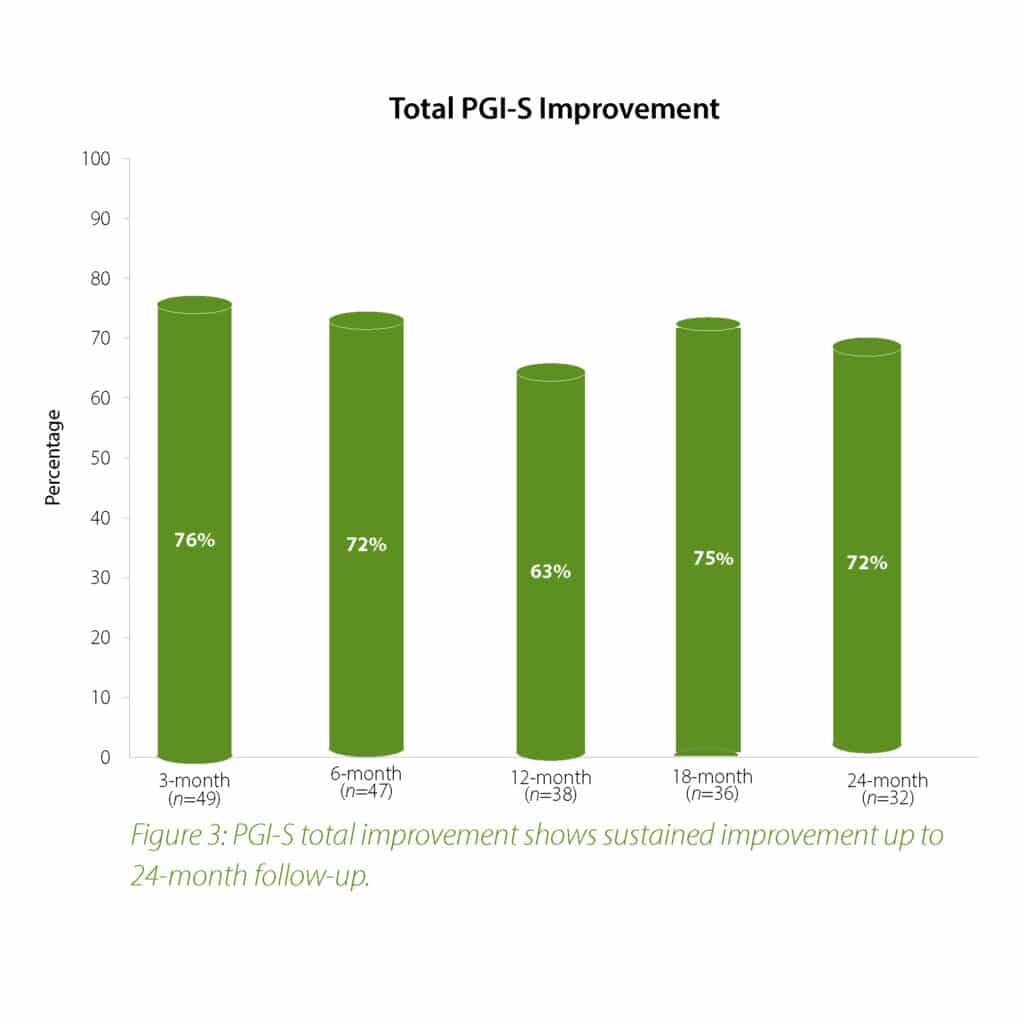
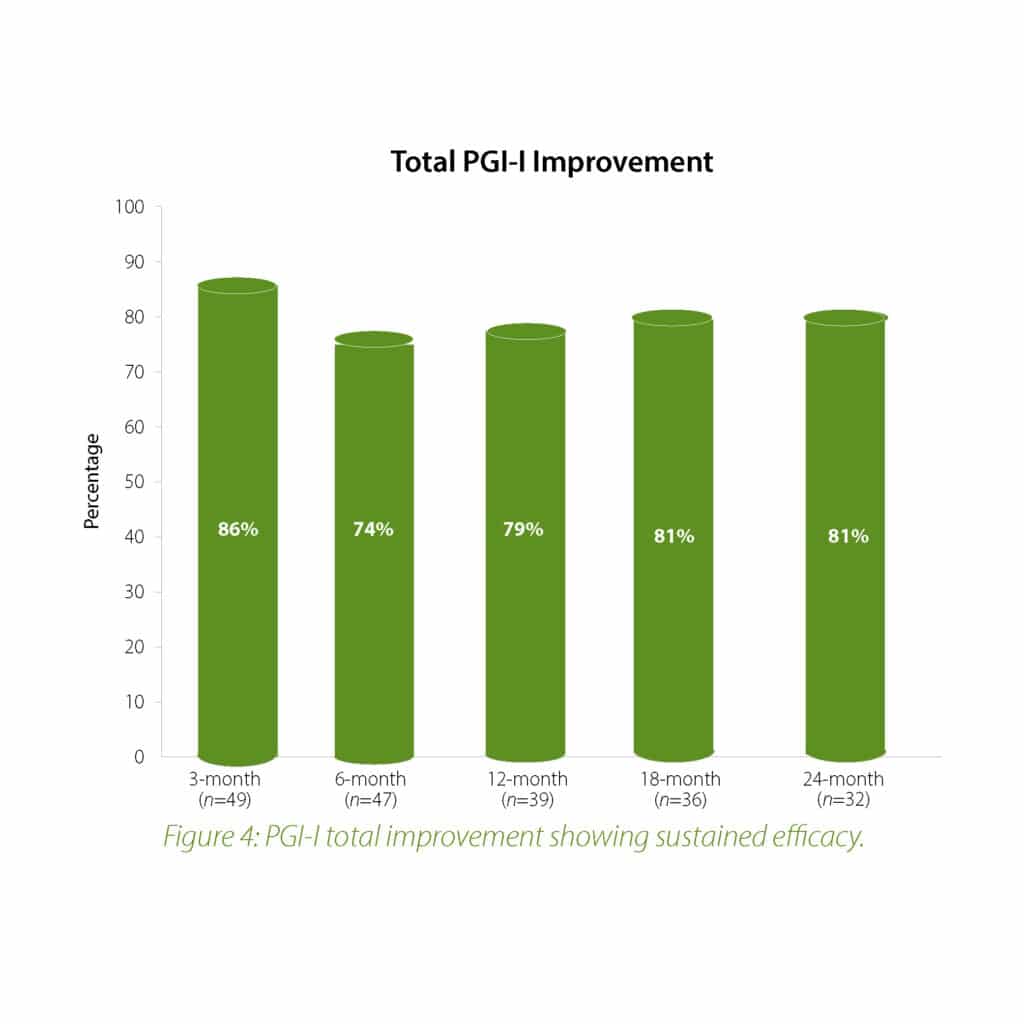
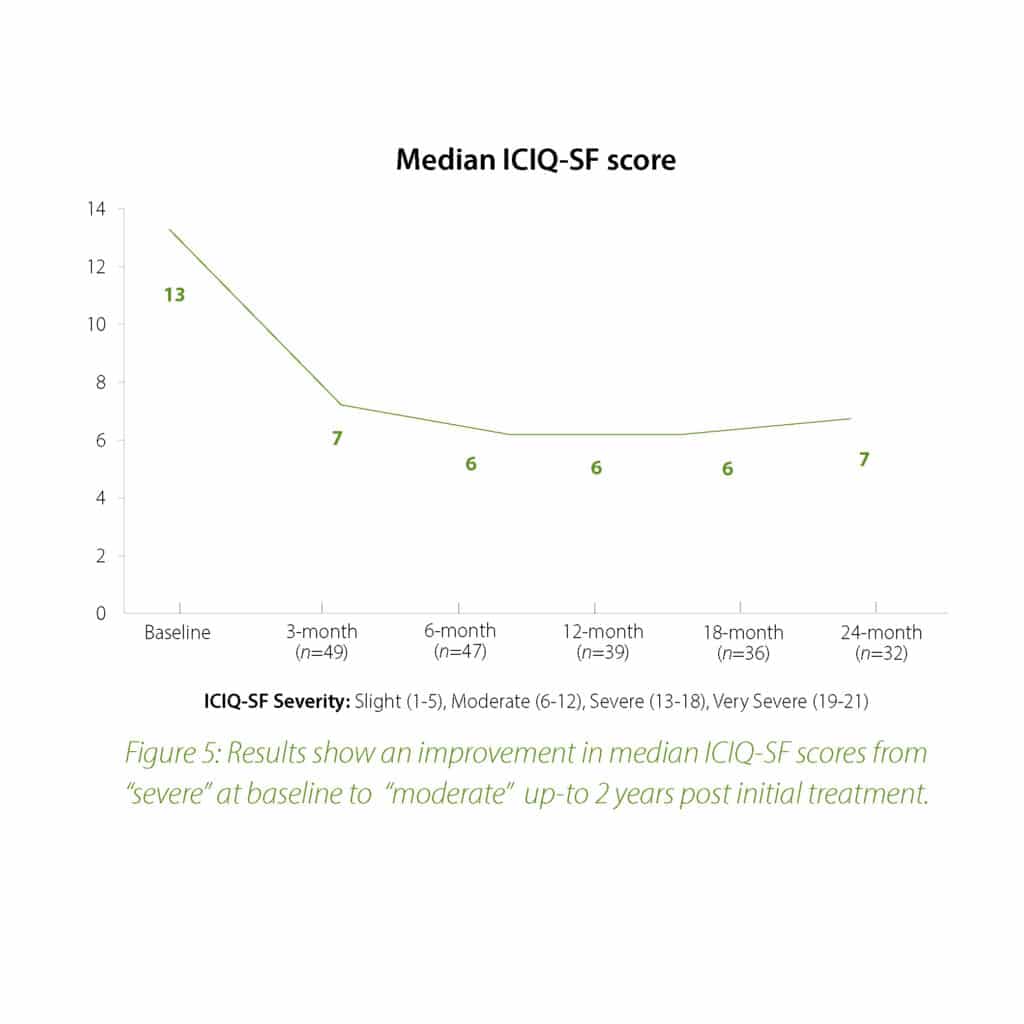
The 2-year follow-up data further confirms the earlier findings of the Urolon clinical effectiveness. Urolon shows to be a safe and effective treatment option for females with mild to moderate SUI who have attempted or failed pelvic floor muscle training. Urolon treatment resulted in improvements in efficacy and patient’s Quality of Life, while being well tolerated. The bioresorption profile of Urolon™ remains a very attractive characteristic as this is a highly sought-after feature.
September 2019
AQLANE Medical™ is pleased to announce that our 12-month clinical data abstract has been accepted for ePoster Presentation at the ICS 2019 Meeting 3-6 September in Gothenburg, Sweden.
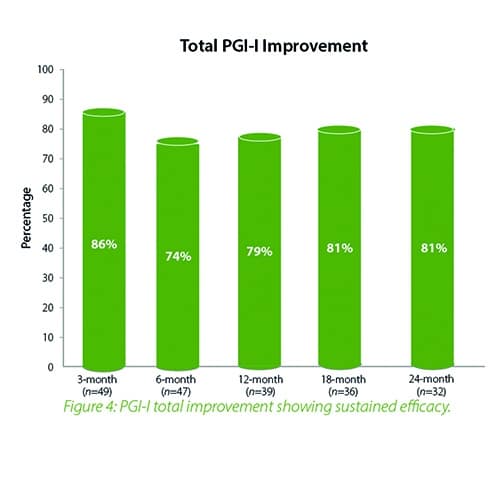
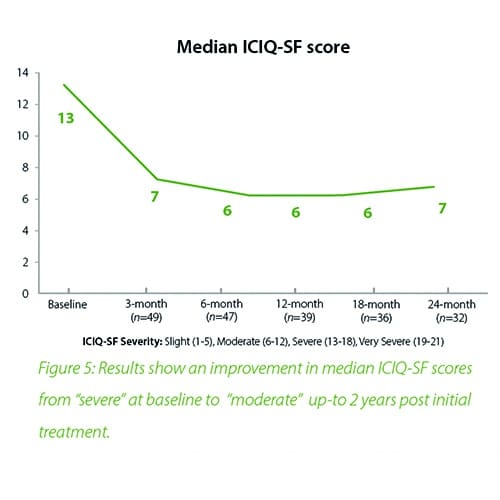
Concluding message: The study shows that the PCL-based bioresorbable urethral implant treatment is safe and effective for women with mild to moderate SUI, resulting in improvements in both SUI severity and QoL. As the study is ongoing (up-to 2 years follow-up), data is subject to change.
Please click here to read the full abstract and here to download the ePoster.
July 2019
In summary, the 18-month analysis shows Urolon™ as a safe and effective treatment option for females with mild to moderate SUI who have attempted or failed pelvic floor muscle training. Results show improvements in both SUI severity and QoL with only 6 subjects that reported treatment-related AE’s which supports a high safety profile. Anecdotal reports from all clinical study sites confirms the performance of Urolon™ as superior in comparison to current urethral bulking agents on the market. The bioresorption profile of Urolon™ is an attractive characteristic and feedback from urologists, gynecologists and urogynecologists alike, suggest this is a highly sought-after feature. As the study is ongoing additional data will be provided at 24-month follow-up to further support the safety and efficacy profile of Urolon™.
April 2019
AQLANE Medical™ is pleased to announce that our 12-month clinical data abstract has been accepted for Oral Presentation at the UKCS 2019 Meeting, 24-26 April in Manchester, United Kingdom.
Please click here to read the full abstract.
November 2018
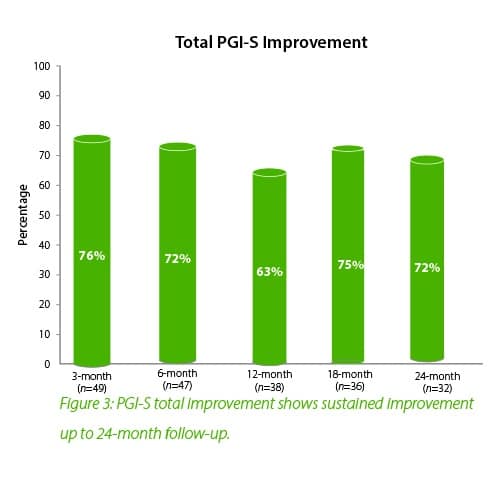
In summary, the 12-month intermediate analysis shows Urolon™ as a safe and effective treatment option for woman with mild to moderate SUI who have attempted or failed pelvic floor muscle training. Results show improvements in SUI severity and QoL (see figure 1-3) with only few mild treatment-related AE’s which supports a high safety profile. The clinical study sites expressed their high satisfaction with the performance of Urolon™. The bioresorption profile of Urolon™ is an attractive characteristic and feedback from urologists, gynecologists and urogynecologists alike, suggest this is a highly sought-after feature of a urethral implant. As the study is ongoing additional data will be provided at 18- and 24-month follow-up to further confirm the safety and efficacy profile of Urolon™.
August 2018
AQLANE Medical™ is pleased to announce that our 6-month clinical data abstract has been accepted for Poster Presentation at the ICS 2018 Meeting 28-31 August in Philadelphia, USA.
Concluding message: QoL measures are an essential end-point for measuring patients perceptions of the effects of incontinence treatments. Improvements in QoL shown here further demonstrate the efficacy of this PCL-based bulking agent. In addition to its efficacy, bioresorption is an attractive safety feature of the PCL-based bulking agent when compared to permanent materials. Preliminary results suggest that the bioresorbable PCL-based bulking agent is a promising safe and effective treatment option, and a valuable addition to the current treatment armament for female patients with mild to moderate SUI, who attempted and failed prior pelvic floor muscle training.
Please click here to read the full abstract and here to download the ePoster.
October 2017
AQLANE Medical™ is pleased to announce that abstract entitled “Treatment with a polycaprolactone-based bioresorbable collagen stimulator (BCS) for mild to moderate SUI” showcasing interim data from our European pivotal trial was accepted for a poster presentation by the EUGA Scientific Committee.
July 2017
AQLANE Medical™ is pleased to announce that patient enrollment is completed and all subjects are treated in their multicenter European clinical trial entitled “The use of a Polycaprolactone Based Bulking Agent for the Treatment of Female Stress Urinary Incontinence (SUI), a Pivotal Trial”.
September 2016
AQLANE Medical™ is pleased to announce the first patient has been enrolled and treated in their multicenter clinical trial entitled “The use of a Polycaprolactone Based Bulking Agent for the Treatment of Female Stress Urinary Incontinence (SUI), a Pivotal Trial”.
July 2016
AQLANE Medical™ received approval to start their multicenter clinical trial in the Netherlands. Following the University Hospital in Antwerp (UZA), Belgium, two Dutch sites will start patient enrollment; the Catharina Hospital in Eindhoven and the Laurentius Hospital in Roermond.
The clinical trial is registered in the Dutch Trial Register (NTR) under number 6002, please click here to read the trial info.
May 2016
AQLANE Medical™ received approval to start their multicenter clinical trial in Belgium. The University Hospital in Antwerp (UZA) is the first hospital to enroll patients into this 2-year study.
September 2015
AQLANE Medical™ initiated a prospective, pivotal trial to study the safety and efficacy of Urolon™, our PCL-based bulking agent for female stress urinary incontinence (SUI). Site selection is completed and patient enrollment is underway.
